Chemistry, 14.02.2020 23:27 bthakkar25
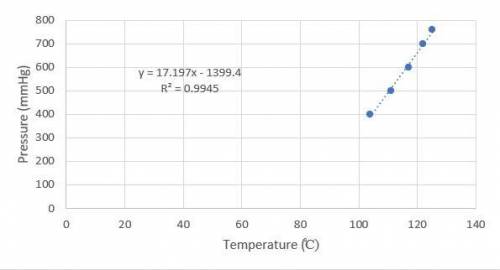
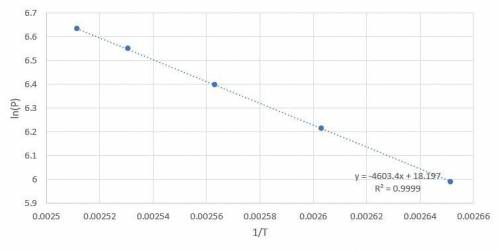
Octane is a liquid component of gasoline. Given the following vapor pressures of octane at various temperatures, estimate the boiling point of octane in Leadville, Colorado, where the atmospheric pressure is 496 mmHg.
a. 400 mmHg at 104oC
b. 500 mmHg at 111oC
c. 600 mmHg at 117oC
d. 700 mmHg at 122oC
e. 760 mmHg at 125oC

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
In any energy conversion, some of the energy is lost to the environment as question 5 options: electrical energy potential energy sound energy thermal energy
Answers: 1

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1

Chemistry, 23.06.2019 00:30
How can you write e method for the experiment of separating sand from water by filtration process? 1-materials 2-steps 3-conclusion also the same for the separating process of water and salt by filtration or distillation. quick because i need to finish my hw
Answers: 2
You know the right answer?
Octane is a liquid component of gasoline. Given the following vapor pressures of octane at various t...
Questions




English, 20.07.2019 03:00






English, 20.07.2019 03:00



Mathematics, 20.07.2019 03:00





Mathematics, 20.07.2019 03:00

Computers and Technology, 20.07.2019 03:00

Chemistry, 20.07.2019 03:00