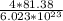Chemistry, 14.02.2020 23:54 andrejr0330jr
Zinc oxide adopts a face-centered cubic arrangement. Both Zinc ions and oxide ions adopt the face-centered cubic arrangement; with the unit cell defined by the cations overlapping the unit cell defined by the anions. What is the mass of one unit cell of zinc oxide?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Is this a scientific model? use complete sentences to explain why or why not. a graphic organizer showing the water cycle i need : ( asap i go it never mind
Answers: 2

Chemistry, 22.06.2019 01:00
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3

Chemistry, 22.06.2019 18:30
How many moles of lead are in 1.50 x 10^12 atoms of lead? could you explain the answer as well and not just give it to me i am refreshing for finals and i need to know how to do it
Answers: 3
You know the right answer?
Zinc oxide adopts a face-centered cubic arrangement. Both Zinc ions and oxide ions adopt the face-ce...
Questions


Biology, 18.09.2019 21:10



English, 18.09.2019 21:10





Chemistry, 18.09.2019 21:10

Biology, 18.09.2019 21:10


Mathematics, 18.09.2019 21:10






Geography, 18.09.2019 21:10