
Chemistry, 15.02.2020 00:32 keyairareid7
How many moles of ions are present in an ideal solution that is produced by dissolving 22.6 g of Cu(NO 3) 2 in 323 g of water?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no2]2 ? second order 3/2 order third order zero order none of the listed answers are correct
Answers: 3

Chemistry, 22.06.2019 07:00
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1

You know the right answer?
How many moles of ions are present in an ideal solution that is produced by dissolving 22.6 g of Cu(...
Questions

Mathematics, 23.08.2020 02:01

Chemistry, 23.08.2020 02:01

English, 23.08.2020 02:01

Biology, 23.08.2020 02:01



Business, 23.08.2020 02:01

Mathematics, 23.08.2020 02:01

Chemistry, 23.08.2020 02:01


World Languages, 23.08.2020 02:01

Arts, 23.08.2020 02:01

Health, 23.08.2020 02:01

History, 23.08.2020 02:01


Mathematics, 23.08.2020 02:01


Biology, 23.08.2020 02:01


Mathematics, 23.08.2020 02:01

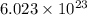 of particles.
of particles.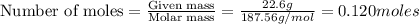
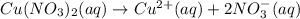
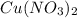 gives = 3 moles of ions
gives = 3 moles of ions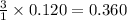 moles of ions.
moles of ions.

