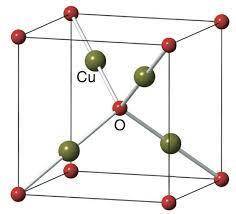
Cuprite, Cu2O, has a body-centered cubic unit cell of oxide anions with four copper cations in a tetrahedral arrangement around the body center oxide. Draw the unit cell in the empty cube. What is the coordination number and shape around the copper cations, and what is the charge on copper? Do the same analysis for the oxide anions.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1


Chemistry, 23.06.2019 01:00
Which of the following is the molecular formula for a simple sugar? a. cooh b. h2o c. oh d. c6h12o6
Answers: 1
You know the right answer?
Cuprite, Cu2O, has a body-centered cubic unit cell of oxide anions with four copper cations in a tet...
Questions



Mathematics, 10.09.2021 22:10


History, 10.09.2021 22:10




Mathematics, 10.09.2021 22:10

Chemistry, 10.09.2021 22:10

History, 10.09.2021 22:10

Mathematics, 10.09.2021 22:10

Physics, 10.09.2021 22:10

Physics, 10.09.2021 22:10

Biology, 10.09.2021 22:10


English, 10.09.2021 22:10



Mathematics, 10.09.2021 22:10