
Chemistry, 15.02.2020 02:28 gamingisfun
At a wastewater treatment plant, FeCl3(s) is added to remove excess phosphate from the effluent. Assume the following reactions occur: FeCl3 ---> Fe3+ + 3Cl- FePO4 ---> Fe3+ + PO4 3␣ The equilibrium constant for the second reaction is Ksp 1⁄4. What concentration of Fe3+ is needed to maintain the phosphate concentration below the limit of 1 mg P/L?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3

Chemistry, 23.06.2019 14:30
The first supersonic flight was in 1947. it was just above the speed of sound. which altitude would you expect captain yeager to have used for his flight
Answers: 3
You know the right answer?
At a wastewater treatment plant, FeCl3(s) is added to remove excess phosphate from the effluent. Ass...
Questions


English, 17.11.2020 02:40


Biology, 17.11.2020 02:40


History, 17.11.2020 02:40




Mathematics, 17.11.2020 02:40



Spanish, 17.11.2020 02:40

Mathematics, 17.11.2020 02:40


Mathematics, 17.11.2020 02:40

Social Studies, 17.11.2020 02:40


Mathematics, 17.11.2020 02:40

Mathematics, 17.11.2020 02:40

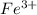 needed is,
needed is, 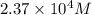
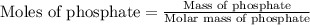
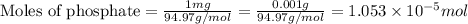
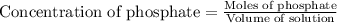
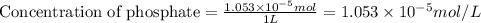
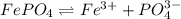
![K_{sp}=[Fe^{3+}][PO_4^{3-}]](/tpl/images/0512/1855/b47eb.png)
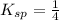
![\frac{1}{4}=[Fe^{3+}]\times 1.053\times 10^{-5}mol/L](/tpl/images/0512/1855/f5443.png)
![[Fe^{3+}]=2.37\times 10^4M](/tpl/images/0512/1855/0a4ad.png)


