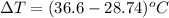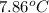A 56-kg hiker is climbing the 828-m-tall Burj Khalifa in Dubai. If the efficiency of converting the energy content of the bars into the work of climbing is 25%, the remaining 75% of the energy released through metabolism is heat released to her body. She eats two energy bars, one of which produces 1.10×103kJ of energy upon metabolizing. Assume that the heat capacity of her body is equal to that for water (75.3 Jmol−1⋅K−1). Calculate the increase in her temperature at the top of the structure.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which element in the third period would you expect to have the larger atomic radius, sodium (na) or sulfur (s)? a. sodium, because it has a higher effective nuclear charge attracting electrons in fewer energy levels. b. sodium, because it has fewer protons attracting electrons in the same energy levels. c. sulfur, because it has more protons attracting electrons in more energy levels. d. sulfur, because it has a higher effective nuclear charge attracting electrons in the same energy levels.
Answers: 2

Chemistry, 21.06.2019 17:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5 m hcl? show all of the work needed to solve this problem. mg (s) + 2hcl (aq) → mgcl2 (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 23.06.2019 00:30
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1
You know the right answer?
A 56-kg hiker is climbing the 828-m-tall Burj Khalifa in Dubai. If the efficiency of converting the...
Questions

Business, 01.12.2020 06:00

Mathematics, 01.12.2020 06:00



World Languages, 01.12.2020 06:00



Chemistry, 01.12.2020 06:00

Social Studies, 01.12.2020 06:00

English, 01.12.2020 06:00

Social Studies, 01.12.2020 06:00



Mathematics, 01.12.2020 06:00


Computers and Technology, 01.12.2020 06:00




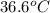 .
. 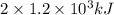
 kJ
kJ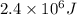 (as 1 kJ = 1000 J)
(as 1 kJ = 1000 J)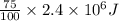
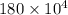 J
J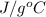
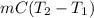
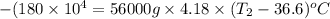
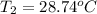
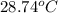 .
.