
Chemistry, 15.02.2020 04:27 lekaje2375
A generic element, G, is composed of two isotopes, 132G and 128G. 132G has a natural abundance of 90% and an isotopic mass of 131.90 amu, and 128G has a natural abundance of 10% and an isotopic mass of 127.90 amu. What is the average atomic mass of this element?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5 m hcl? show all of the work needed to solve this problem. mg (s) + 2hcl (aq) → mgcl2 (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1
You know the right answer?
A generic element, G, is composed of two isotopes, 132G and 128G. 132G has a natural abundance of 90...
Questions

Social Studies, 17.10.2019 17:30


Mathematics, 17.10.2019 17:30





Health, 17.10.2019 17:30

Mathematics, 17.10.2019 17:30





Biology, 17.10.2019 17:30


Mathematics, 17.10.2019 17:30

Mathematics, 17.10.2019 17:30


History, 17.10.2019 17:30

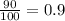
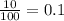
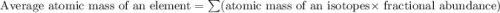
![A=\sum[(131.90\times 0.9)+(127.90\times 0.1)]=131.5amu](/tpl/images/0512/2891/2177c.png)


