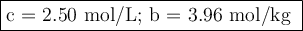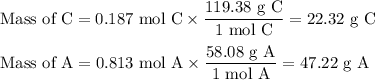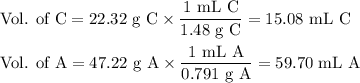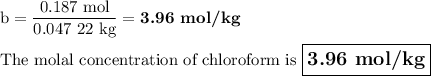Chemistry, 16.02.2020 02:36 keegandudley
A chemist combined chloroform (CHCl3) and acetone (C3H6O) to create a solution where the mole fraction of chloroform is 0.187. The densities of chloroform and acetone are 1.48 g/mL and 0.791 g/mL, respectively.
Calculate the molarity and molality of the solution.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1
You know the right answer?
A chemist combined chloroform (CHCl3) and acetone (C3H6O) to create a solution where the mole fracti...
Questions


Mathematics, 25.01.2021 18:20


History, 25.01.2021 18:20

Mathematics, 25.01.2021 18:20


Mathematics, 25.01.2021 18:20

Mathematics, 25.01.2021 18:20

Geography, 25.01.2021 18:20


Mathematics, 25.01.2021 18:20

Mathematics, 25.01.2021 18:20

English, 25.01.2021 18:20

History, 25.01.2021 18:20

Mathematics, 25.01.2021 18:20

Biology, 25.01.2021 18:20

Physics, 25.01.2021 18:20

Computers and Technology, 25.01.2021 18:20

Mathematics, 25.01.2021 18:20