Chemistry, 16.02.2020 19:41 queenkimm26
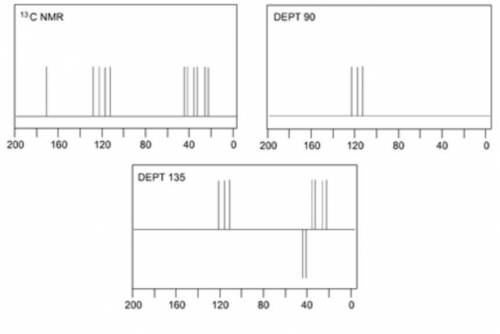
When an unknown amine reacts with an unknown acid chloride, an amide with a molecular mass of 163 g/mol (M = 163 m/z) is formed. In the infrared spectrum, important absorptions appear at 1661, 750 and 690 cm–1. The 13C NMR and DEPT spectra are provided below. Draw the structure of the product as the resonance contributor lacking any formal charges.

Answers: 1


Another question on Chemistry

Chemistry, 20.06.2019 18:04
In which of the following belong to a category called the main group of elements? nonmetals. transition elements. halogens. alkali metals. it can be more than one answer!
Answers: 3

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 23.06.2019 04:00
How much energy is required to vaporize 2 kg of copper? a 4730 kj b 207kj c 9460 kj d 414kj
Answers: 1

Chemistry, 23.06.2019 10:00
Why sncl2 is solid while sncl4 is liquid at room temprature explain it in easy way
Answers: 1
You know the right answer?
When an unknown amine reacts with an unknown acid chloride, an amide with a molecular mass of 163 g/...
Questions



Mathematics, 28.01.2021 23:00


Mathematics, 28.01.2021 23:00




Mathematics, 28.01.2021 23:00

English, 28.01.2021 23:00

Social Studies, 28.01.2021 23:00


Mathematics, 28.01.2021 23:00



Mathematics, 28.01.2021 23:00

English, 28.01.2021 23:00


Health, 28.01.2021 23:00

Chemistry, 28.01.2021 23:00