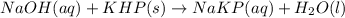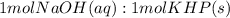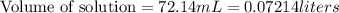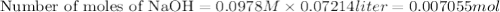Chemistry, 17.02.2020 06:06 asdf334asdf334
Answer the following questions based on the reaction below: NaOH(aq) + KHP(s) --> NaKP(aq)+H2O(I)
A 1.864 g sample of impure KHP was titrated with a 0.0978 M solution of NaOH. To completely react the KHP in the sample, 72.14 mL of base was needed. KHP (potassium hydrogen phthalate, 204.23 g/mol)
a.) How many grams of KHP were in the unknown sample?
b.) What is the percentage of KHP in the unknown sample?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1



Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2
You know the right answer?
Answer the following questions based on the reaction below: NaOH(aq) + KHP(s) --> NaKP(aq)+H2O(I)...
Questions



Physics, 24.09.2021 20:20




Mathematics, 24.09.2021 20:20


History, 24.09.2021 20:20



Mathematics, 24.09.2021 20:30

Mathematics, 24.09.2021 20:30


Mathematics, 24.09.2021 20:30



Mathematics, 24.09.2021 20:30


Mathematics, 24.09.2021 20:40