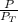A mixture of ethanol and 1−propanol behaves ideally at 36°C and is in equilibrium with its vapor. If the mole fraction of ethanol in the solution is 0.65, calculate its mole fraction in the vapor phase at this temperature. (The vapor pressures of pure ethanol and 1−propanol at 36°C are 108 and 40.0 mmHg, respectively.)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Which of the following statements is false regarding aromaticity? a. the compound must be cyclic b. the compound must be fully conjugated c. the compound must be planar d.the number of electrons in the pi system must satisfy the hückel 4n+2 rule e. the compound must have a neutral charge
Answers: 2

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 13:00
Adepositional also feature that forms where a stream enters a lake or an ocean is a
Answers: 2
You know the right answer?
A mixture of ethanol and 1−propanol behaves ideally at 36°C and is in equilibrium with its vapor. If...
Questions


Social Studies, 19.07.2021 14:00

English, 19.07.2021 14:00

Mathematics, 19.07.2021 14:00

English, 19.07.2021 14:00

Physics, 19.07.2021 14:00

Mathematics, 19.07.2021 14:00

Mathematics, 19.07.2021 14:00

Engineering, 19.07.2021 14:00


Social Studies, 19.07.2021 14:00


Chemistry, 19.07.2021 14:00



Biology, 19.07.2021 14:00

English, 19.07.2021 14:00

English, 19.07.2021 14:00

English, 19.07.2021 14:00

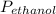 = 0.65 × 108 mmHg
= 0.65 × 108 mmHg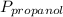 = 0.35× 40.0 mmHg
= 0.35× 40.0 mmHg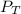 = 70.2 + 4.9 = 75.1 mmHg
= 70.2 + 4.9 = 75.1 mmHg