Chemistry, 17.02.2020 17:27 hiplikedyani
Calculate the amount of heat released in the combustion of 10.5 grams of Al with 3 grams of O2 to form Al2O3(s) at 25°C and 1 atm. ΔHfAl2O3(s) = −1676 kJ/mol HINT: What does ΔHfAl2O3(s) mean?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

You know the right answer?
Calculate the amount of heat released in the combustion of 10.5 grams of Al with 3 grams of O2 to fo...
Questions


Mathematics, 30.05.2020 03:04





Chemistry, 30.05.2020 03:04

Biology, 30.05.2020 03:04

Mathematics, 30.05.2020 03:04

Mathematics, 30.05.2020 03:04



Social Studies, 30.05.2020 03:05

Mathematics, 30.05.2020 03:05

Biology, 30.05.2020 03:05


History, 30.05.2020 03:05

Chemistry, 30.05.2020 03:05


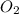 .
.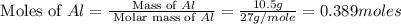
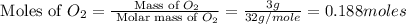
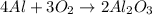
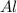
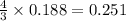 moles of
moles of 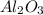
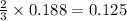 moles of
moles of