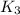The following two-step process has equilibrium constants K1 and K2. Step 1: H2(g) + ICl(g) → HI(g) + HCl(g) K1 Step 2: HI(g) + ICl(g) → HCl(g) + I2(g) K2 Overall : H2(g) + 2ICl(g) → 2HCl(g) + I2(g) K3 What is the expression for the equilibrium constants for the overall reaction, K3?

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 18:30
Two people each hold the end of a rope and create waves by moving their arms up and down. this wave is best classified as a transverse wave because a) both the rope particles and the wave are moving in the same direction. b) the wave is moving up and down as the particles of the rope move horizontally. c) the wave is moving horizontally as the particles of the rope move up and down. eliminate d) the wave is moving in a parallel direction with the motion of the person's arms.
Answers: 3

Chemistry, 23.06.2019 00:00
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3
You know the right answer?
The following two-step process has equilibrium constants K1 and K2. Step 1: H2(g) + ICl(g) → HI(g) +...
Questions



English, 01.07.2020 16:01




Mathematics, 01.07.2020 16:01

Mathematics, 01.07.2020 16:01




Mathematics, 01.07.2020 16:01

Mathematics, 01.07.2020 16:01


Mathematics, 01.07.2020 16:01

SAT, 01.07.2020 16:01

Physics, 01.07.2020 16:01

Mathematics, 01.07.2020 16:01


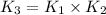
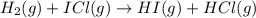 ;
; 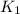
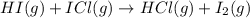 ;
; 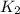
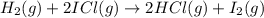 ;
;