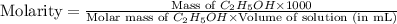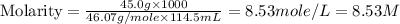Chemistry, 17.02.2020 19:39 nikki987654
The density of a 45.0 mass % solution of ethanol (C2H5OH) in water is 0.873 g/mL. What is the molarity of the solution?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 23.06.2019 00:50
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3

Chemistry, 23.06.2019 01:00
What two factors can affect the properties of a hydrocarbon? a. the number of its carbon atoms and the number of single bonds b. the number of its carbon atoms and the arrangement of its atoms c. the arrangement of its atoms and the number of its double bonds
Answers: 1

Chemistry, 23.06.2019 11:00
Afraction can be converted to a decimal by dividing the denominator into the numerator. use a calculator. divide to convert the fractions from part a to decimals. then describe the pattern you see in the decimal.
Answers: 3
You know the right answer?
The density of a 45.0 mass % solution of ethanol (C2H5OH) in water is 0.873 g/mL. What is the molari...
Questions

Social Studies, 04.02.2020 04:55


Mathematics, 04.02.2020 04:55

Mathematics, 04.02.2020 04:55


Mathematics, 04.02.2020 04:55

Biology, 04.02.2020 04:55


Geography, 04.02.2020 04:55




Health, 04.02.2020 04:55

Mathematics, 04.02.2020 04:55

English, 04.02.2020 04:55

Mathematics, 04.02.2020 04:55

Chemistry, 04.02.2020 04:55

Mathematics, 04.02.2020 04:55

English, 04.02.2020 04:55

Mathematics, 04.02.2020 04:55

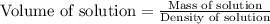
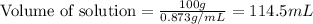
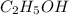 = 45.0 g
= 45.0 g