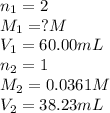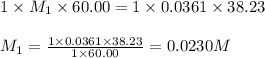Chemistry, 17.02.2020 19:32 Chrisis9987
A 60.00-mL sample of a weak acid is titrated with 0.0361 M NaOH. At the endpoint, it is found that 38.23 mL of titrant was used. What was the concentration of the weak acid?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1


Chemistry, 23.06.2019 06:20
Type the correct answer in each box.balance the chemical equation.__ n203 ➡️ __ n2 +__ o2
Answers: 1

Chemistry, 23.06.2019 10:30
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3
You know the right answer?
A 60.00-mL sample of a weak acid is titrated with 0.0361 M NaOH. At the endpoint, it is found that 3...
Questions

Mathematics, 04.02.2021 07:30


Mathematics, 04.02.2021 07:30

Physics, 04.02.2021 07:30




Mathematics, 04.02.2021 07:30

Mathematics, 04.02.2021 07:30

Mathematics, 04.02.2021 07:30

Mathematics, 04.02.2021 07:30



Mathematics, 04.02.2021 07:30

History, 04.02.2021 07:30



Mathematics, 04.02.2021 07:30


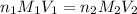
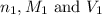 are the n-factor, molarity and volume of acid which is weak acid
are the n-factor, molarity and volume of acid which is weak acid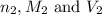 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.