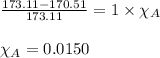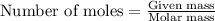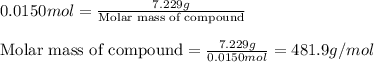Chemistry, 17.02.2020 20:19 justin5647
In a laboratory experiment, students synthesized a new compound and found that when 7.229 grams of the compound were dissolved in 207.8 grams of chloroform, the vapor pressure of the solution was 170.51 mm Hg. The compound was also found to be nonvolatile and a non-electrolyte. What is the molecular weight of this compound?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 09:00
Look at the spectrums of a star moving towards earth and a motionless star. which of these is a correct inference that can be draw from the observation of the two spectrums? (2 points) the spectrum of a motionless star is difficult to be viewed separately using oridinary telescopes. the spectrum of a motionless star is identical to the spectrum of a star which moves towards earth. the spectrum of a star shifts towards the red region when the star moves towards earth. the spectrum of a star shifts towards the blue region when the star moves towards earth.
Answers: 2

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

You know the right answer?
In a laboratory experiment, students synthesized a new compound and found that when 7.229 grams of t...
Questions

English, 03.12.2019 09:31



English, 03.12.2019 09:31




Biology, 03.12.2019 09:31

Mathematics, 03.12.2019 09:31

Mathematics, 03.12.2019 09:31

History, 03.12.2019 09:31

English, 03.12.2019 09:31




Mathematics, 03.12.2019 09:31

Mathematics, 03.12.2019 09:31


World Languages, 03.12.2019 09:31

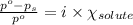
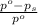 = relative lowering in vapor pressure
= relative lowering in vapor pressure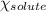 = mole fraction of solute = ?
= mole fraction of solute = ?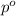 = vapor pressure of pure chloroform = 173.11 mmHg
= vapor pressure of pure chloroform = 173.11 mmHg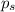 = vapor pressure of solution = 170.51 mmHg
= vapor pressure of solution = 170.51 mmHg