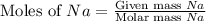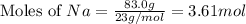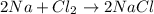Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1
You know the right answer?
How many moles of NaCl will be produced from 83.0g of Na, assuming Cl2 is available in excess?...
Questions







Mathematics, 13.11.2019 20:31











Mathematics, 13.11.2019 20:31


Mathematics, 13.11.2019 20:31

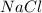 produced will be, 3.61 moles.
produced will be, 3.61 moles.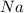 = 83.0 g
= 83.0 g