
Chemistry, 17.02.2020 22:09 carolinasoto
A chemistry student needs 65.0g heptane for an experiment. He has available 20.0g of a 38.1% w/w solution of heptane in chloroform. Calculate the mass of solution the student should use.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1
You know the right answer?
A chemistry student needs 65.0g heptane for an experiment. He has available 20.0g of a 38.1% w/w sol...
Questions

Mathematics, 05.03.2021 03:00

Computers and Technology, 05.03.2021 03:00


Mathematics, 05.03.2021 03:00

History, 05.03.2021 03:00

Chemistry, 05.03.2021 03:00

Social Studies, 05.03.2021 03:00

Mathematics, 05.03.2021 03:00

Mathematics, 05.03.2021 03:00

Mathematics, 05.03.2021 03:00



Mathematics, 05.03.2021 03:00

Mathematics, 05.03.2021 03:00

Mathematics, 05.03.2021 03:00


Mathematics, 05.03.2021 03:00

History, 05.03.2021 03:00

Computers and Technology, 05.03.2021 03:00

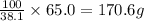 of solution
of solution

