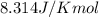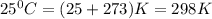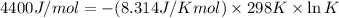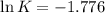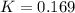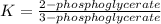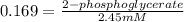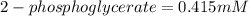The value of Δ G ° ' for the conversion of 3-phosphoglycerate to 2-phosphoglycerate (2PG) is + 4.40 kJ/mol . If the concentration of 3-phosphoglycerate at equilibrium is 2.45 mM , what is the concentration of 2-phosphoglycerate? Assume a temperature of 25.0 ° C .

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 22:30
Which of these statements best explains why space exploration should be encouraged? it prepares humans to live without oxygen. it dispel myths about objects in space. it prevents comets and asteroids from striking earth. it creates technology to absorb harmful radiations in space.
Answers: 1

Chemistry, 23.06.2019 02:00
Now look at the segment of the graph between the two data points marked with black squares. describe how the boiling point and melting point plots behave between these points. be as specific as possible.
Answers: 1
You know the right answer?
The value of Δ G ° ' for the conversion of 3-phosphoglycerate to 2-phosphoglycerate (2PG) is + 4.40...
Questions




Mathematics, 23.07.2019 18:00


Mathematics, 23.07.2019 18:00

Social Studies, 23.07.2019 18:00


Mathematics, 23.07.2019 18:00





Computers and Technology, 23.07.2019 18:00

History, 23.07.2019 18:00

History, 23.07.2019 18:00

Chemistry, 23.07.2019 18:00



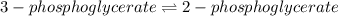
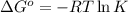
 = Standard Gibbs free energy = +4.40 kJ/mol = 4400 J/mol (Conversion factor: 1kJ = 1000J)
= Standard Gibbs free energy = +4.40 kJ/mol = 4400 J/mol (Conversion factor: 1kJ = 1000J)