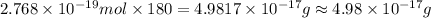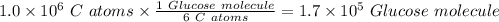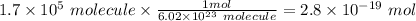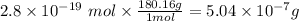Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1


Chemistry, 22.06.2019 18:50
At stp, which substance is the best conductor of electricity? a. nitrogen b. neon c. sulfur d. silver
Answers: 1
You know the right answer?
Calculate the mass of glucose C6H12O6 that contains a million ×1.0106 carbon atoms. Be sure your ans...
Questions

Mathematics, 05.03.2021 21:00


Mathematics, 05.03.2021 21:00



Mathematics, 05.03.2021 21:00

Mathematics, 05.03.2021 21:00


English, 05.03.2021 21:00

English, 05.03.2021 21:00



Mathematics, 05.03.2021 21:00




History, 05.03.2021 21:00


Mathematics, 05.03.2021 21:00

Mathematics, 05.03.2021 21:00

 carbon atoms is
carbon atoms is 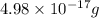 .
.
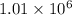 will be in N molecules of glucose:
will be in N molecules of glucose: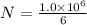 molecules of glucose
molecules of glucose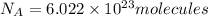
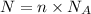
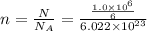
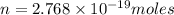
 moles of glucose;
moles of glucose;