
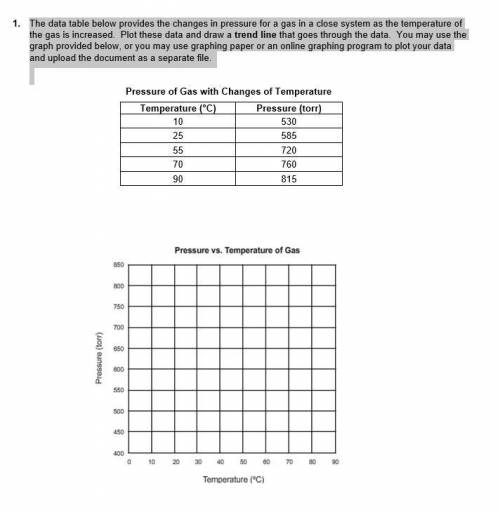
1. The data table below provides the changes in pressure for a gas in a close system as the temperature of the gas is increased. Plot these data and draw a trend line that goes through the data. You may use the graph provided below, or you may use graphing paper or an online graphing program to plot your data and upload the document as a separate file.
• Calculate the slope of the trend line including the units. Be sure to show all steps of your calculations.
• How are the variables of temperature and pressure related to one another?
• Use the trend line to determine what will be the gas pressure at 0°C.
i have attached a picture that shows the data tables

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 21:30
What is the correct name for the compound cocl3? a) cobalt(i) chloride b) cobalt(i) chlorate c) cobalt(ii) chlorate d) cobalt(iii) chloride
Answers: 1

Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1

You know the right answer?
1. The data table below provides the changes in pressure for a gas in a close system as the temperat...
Questions

Mathematics, 05.10.2020 15:01



History, 05.10.2020 15:01

Computers and Technology, 05.10.2020 15:01


Mathematics, 05.10.2020 15:01


History, 05.10.2020 15:01

Mathematics, 05.10.2020 15:01

Mathematics, 05.10.2020 15:01

English, 05.10.2020 15:01

Social Studies, 05.10.2020 15:01



Biology, 05.10.2020 15:01


Chemistry, 05.10.2020 15:01

Mathematics, 05.10.2020 15:01

Computers and Technology, 05.10.2020 15:01



