Chemistry, 18.02.2020 01:46 SkylarAaliyah
Calculate the frequency of the light emitted by a hydrogen atom during a transition of its electron from the n = 4 to the n = 1 principal energy level. Recall
En = –2.18 x 10–18 J(1/n2)
a. 3.08 x 1015/s
b. 1.03 x 108/s
c. 2.06 x 1014/s
d. 1.35 x 10–51/s
e. 8.22 x 1014/s

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
List the two type of transporst that the cell in orde to transport molecules acroos the membrane
Answers: 1

Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2


Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3
You know the right answer?
Calculate the frequency of the light emitted by a hydrogen atom during a transition of its electron...
Questions


History, 21.09.2019 13:30


History, 21.09.2019 13:30

Mathematics, 21.09.2019 13:30

English, 21.09.2019 13:30

Mathematics, 21.09.2019 13:30

Mathematics, 21.09.2019 13:30


English, 21.09.2019 13:30


English, 21.09.2019 13:30

Mathematics, 21.09.2019 13:30


Mathematics, 21.09.2019 13:30


Social Studies, 21.09.2019 13:30



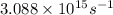
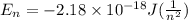
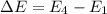
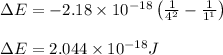
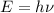
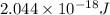
 = frequency of the light = ?
= frequency of the light = ?