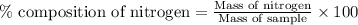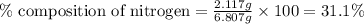Chemistry, 18.02.2020 03:15 jazzycintron14
A 6.807g sample of an unknown compound, composed only of carbon, hydrogen, and nitrogen, produced 13.3g of CO2 and 9.52g of H2O in a combustion analysis. What is the mass percent composition of nitrogen of the unknown compound?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2

Chemistry, 23.06.2019 02:00
What causes the appearance of lines in a emission spectrum
Answers: 1

You know the right answer?
A 6.807g sample of an unknown compound, composed only of carbon, hydrogen, and nitrogen, produced 13...
Questions

Geography, 25.06.2019 12:00


Mathematics, 25.06.2019 12:00

English, 25.06.2019 12:00

Geography, 25.06.2019 12:00

Mathematics, 25.06.2019 12:00


History, 25.06.2019 12:00

History, 25.06.2019 12:00





History, 25.06.2019 12:00

Biology, 25.06.2019 12:00

Biology, 25.06.2019 12:00

Mathematics, 25.06.2019 12:00


Health, 25.06.2019 12:00

Biology, 25.06.2019 12:00

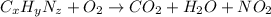
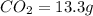
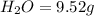
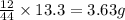 of carbon will be contained.
of carbon will be contained.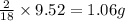 of hydrogen will be contained.
of hydrogen will be contained.