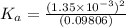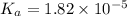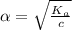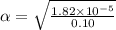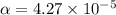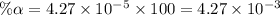Chemistry, 18.02.2020 05:22 Mangolinux7173
Start with 100.00 mL of 0.10 M acetic acid, CH3COOH. The solution has a pH of 2.87 at 25 oC. a) Calculate the Ka of acetic acid at 25 oC. b) Determine the percent dissociation for the solution.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1


Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2
You know the right answer?
Start with 100.00 mL of 0.10 M acetic acid, CH3COOH. The solution has a pH of 2.87 at 25 oC. a) Calc...
Questions

Mathematics, 31.07.2019 04:00



Mathematics, 31.07.2019 04:00



Mathematics, 31.07.2019 04:00



Chemistry, 31.07.2019 04:00

English, 31.07.2019 04:10

Chemistry, 31.07.2019 04:10




Mathematics, 31.07.2019 04:10

Mathematics, 31.07.2019 04:10

Computers and Technology, 31.07.2019 04:10


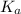 of acetic acid at
of acetic acid at 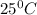 is
is 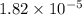
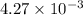
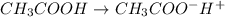
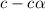
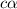
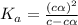
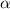 = ?
= ?![pH=-log[H^+]](/tpl/images/0513/9443/15713.png)
![2.87=-log[H^+]](/tpl/images/0513/9443/3a07c.png)
![[H^+]=1.35\times 10^{-3}M](/tpl/images/0513/9443/01bcb.png)
![[CH_3COO^-]=1.35\times 10^{-3}M](/tpl/images/0513/9443/0a4ad.png)
![[CH_3COOH]=(0.10M-1.35\times 10^{-3}=0.09806M](/tpl/images/0513/9443/5420f.png)