
The rate law for the reaction
2NO(g) + Cl2(g) → 2NOCl(g)
is given by R = k[NO][Cl2]
If the following is the mechanism for the reaction, NO(g) + Cl2(g) → NOCl2(g) NOCl2(g) + NO(g) → 2NOCl(g)
which of the following statements accurately describes this reaction? Check all that apply.
-2nd order reaction
-The first step is the slow step.
-Doubling [NO] would quadruple the rate.
-Cutting [Cl2] in half would decrease the rate by a factor of two.
-The molecularity of the first step is 1.
-Both steps are termolecular.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2
You know the right answer?
The rate law for the reaction
2NO(g) + Cl2(g) → 2NOCl(g)
is given by R = k...
2NO(g) + Cl2(g) → 2NOCl(g)
is given by R = k...
Questions

English, 24.01.2020 03:31

Mathematics, 24.01.2020 03:31


Biology, 24.01.2020 03:31

English, 24.01.2020 03:31

Mathematics, 24.01.2020 03:31

Mathematics, 24.01.2020 03:31


Mathematics, 24.01.2020 03:31




History, 24.01.2020 03:31


Mathematics, 24.01.2020 03:31

Computers and Technology, 24.01.2020 03:31


Mathematics, 24.01.2020 03:31

Biology, 24.01.2020 03:31

Mathematics, 24.01.2020 03:31

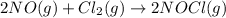
![k[NO][Cl_{2}]](/tpl/images/0514/1761/8cd0b.png)
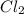 . Since, power of the concentrations of each of these is equal to 1. Therefore, order of the reaction is equal to 1 + 1 = 2.
. Since, power of the concentrations of each of these is equal to 1. Therefore, order of the reaction is equal to 1 + 1 = 2.

