
Chemistry, 18.02.2020 17:15 griffinadrianne946
Diamond and graphite are two crystalline forms of carbon. At 1 atm and 25∘C diamond changes to graphite so slowly that the enthalpy change of the process must be obtained indirectly. Determine △ Hrxn for C(diamond) → C(graphite) with equations from the following list: (1) C(dianond)+O2(g)⟶CO2(g)ΔH=−395.4kJ (2) 2CO2(g)⟶2CO(g)+O2(g)ΔH=566.0kJ, (3) C(graphite)+O2(g)→CO2(g)ΔH=−393.5kJ , (4) 2CO(g)⟶C(graphite)+CO2(g)ΔH=−172.5k J.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:40
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2

Chemistry, 23.06.2019 02:00
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1

Chemistry, 23.06.2019 06:30
The polarity of an oxygen-hydrogen bond is higher than the polarity of a nitrogen-hydrogen bond, allowing amines to be more soluble than alcohols.
Answers: 3

Chemistry, 23.06.2019 10:00
State the effect on the concentration of the clo- ion when there is a decrease in the concentration of the oh- ion
Answers: 1
You know the right answer?
Diamond and graphite are two crystalline forms of carbon. At 1 atm and 25∘C diamond changes to graph...
Questions



Mathematics, 17.01.2021 20:30

Mathematics, 17.01.2021 20:30

Advanced Placement (AP), 17.01.2021 20:30


Mathematics, 17.01.2021 20:30

History, 17.01.2021 20:30

Mathematics, 17.01.2021 20:30

Mathematics, 17.01.2021 20:30


Mathematics, 17.01.2021 20:30

Mathematics, 17.01.2021 20:30



Biology, 17.01.2021 20:30



Health, 17.01.2021 20:30

Mathematics, 17.01.2021 20:30

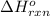 for the reaction is -1.9 kJ.
for the reaction is -1.9 kJ.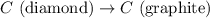
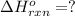
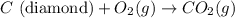
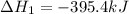
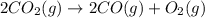
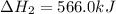 ( × 2)
( × 2)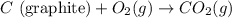
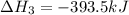
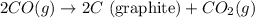
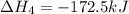 ( × 2)
( × 2)![\Delta H^o_{rxn}=[1\times (\Delta H_1)]+[2\times \Delta H_2]+[1\times (\Delta H_3)]+[2\times \Delta H_4]](/tpl/images/0514/1921/04537.png)
![\Delta H^o_{rxn}=[(1\times (-395.4))+(2\times (566.0))+(1\times (-393.5))+(2\times (-172.5))]=-1.9kJ](/tpl/images/0514/1921/ca977.png)


