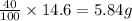Chemistry, 18.02.2020 18:23 DisneyGirl11
There is 60.0% oxygen by mass in a certain compound of sulfur and oxygen. Percent by mass is a ratio of the number of grams of a particular component to 100 g of the total sample. How many grams of sulfur are in a 14.6 g sample of the compound?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:40
The formation of a solid, also known as a is an indication of a chemical change. precipitate particulate particle powder
Answers: 3

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2

Chemistry, 23.06.2019 00:00
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1
You know the right answer?
There is 60.0% oxygen by mass in a certain compound of sulfur and oxygen. Percent by mass is a ratio...
Questions

Business, 18.03.2021 03:10

Mathematics, 18.03.2021 03:10

History, 18.03.2021 03:10


History, 18.03.2021 03:10

History, 18.03.2021 03:10


Mathematics, 18.03.2021 03:10

Mathematics, 18.03.2021 03:10





History, 18.03.2021 03:10




Mathematics, 18.03.2021 03:10