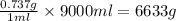Chemistry, 18.02.2020 18:33 gottapass62
A certain molecular compound has a solubility in hexane of at . Calculate the mass of that's dissolved in of a saturated solution of in hexane at this temperature. Be sure your answer has the correct unit symbol and significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Calculate the expected ph values of the buffer systems from the experiments (a,b,c,d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 23.06.2019 00:30
There are approximately 15 milliliters (ml) in 1 tablespoon (tbsp). what expression can be used to find the approximate number of milliliters in 3 tbsp?
Answers: 1

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 1

Chemistry, 23.06.2019 04:50
The diagin dilutepage 6 of 12a6a5(a)fluorine, chlorine, bromine and iodine are placed in the same group of theperiodic table.state the common name used to describe elements in this group.(i)state the group in which the elements are placed and explain whythey are placed in that group.(ii)which of the above named elements is a solid at roomtemperature and pressure?
Answers: 2
You know the right answer?
A certain molecular compound has a solubility in hexane of at . Calculate the mass of that's dissolv...
Questions

Geography, 10.12.2020 06:10


Mathematics, 10.12.2020 06:10


Mathematics, 10.12.2020 06:10



Mathematics, 10.12.2020 06:10

Mathematics, 10.12.2020 06:10

History, 10.12.2020 06:10


History, 10.12.2020 06:10

Mathematics, 10.12.2020 06:10


Chemistry, 10.12.2020 06:10


Mathematics, 10.12.2020 06:20

Mathematics, 10.12.2020 06:20