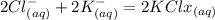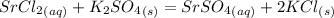When an aqueous solution of strontium chloride is added to an aqueous solution of potassium sulfate, a precipitation reaction occurs. Write the balanced net ionic equation of the reaction. Include charges on the ions, where applicable. Include coefficients only when they are different than ?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 22:30
What is a number added in front of a formula in order to balance the equation
Answers: 1

Chemistry, 22.06.2019 23:30
Aweight lifter raises a 1600 n barbell to a height of 2.0 meters. how much work was done? w = fd a) 30 joules b) 3000 joules c) 320 joules d) 3200 joules
Answers: 2

Chemistry, 23.06.2019 01:30
If a particle has z = 25 and 23 electrons, what is its charge?
Answers: 2

Chemistry, 23.06.2019 03:00
Use the half-reactions of the reaction au(oh)3 + hi -> au +i2 +h2o to answer the questions
Answers: 1
You know the right answer?
When an aqueous solution of strontium chloride is added to an aqueous solution of potassium sulfate,...
Questions

Mathematics, 11.05.2021 08:30


Law, 11.05.2021 08:30


Law, 11.05.2021 08:30

Mathematics, 11.05.2021 08:30

History, 11.05.2021 08:30



Mathematics, 11.05.2021 08:30


Mathematics, 11.05.2021 08:30

History, 11.05.2021 08:30

Physics, 11.05.2021 08:30


Business, 11.05.2021 08:30

Mathematics, 11.05.2021 08:30

Mathematics, 11.05.2021 08:30

Mathematics, 11.05.2021 08:30