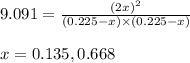Chemistry, 18.02.2020 18:28 happysage12
At a certain temperature the equilibrium constant, Kc, equals 0.11 for the reaction:
2 ICl(g) ? I2(g) + Cl2(g).
What is the equilibrium concentration of ICl if 0.45 mol of I2 and 0.45 mol of Cl2 are initially mixed in a 2.0-L flask?
a.) 0.17 M
b.) 0.27 M
c.) 0.34 M
d.) 0.14 M

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Does anyone know a lot about how to: - calculate mass of magnesium metal - calculate the actual yield of magnesium oxide - calculate the theoretical yield of mgo - calculate the percent yield of mgo - determine the percent yield of mgo - determine the average percent yield of mgo i had to do an online lab and its asking these questions but i have no idea where to start or how to be able to find these things. i can post the chart of the data from the lab or if you can tell me exactly how i can find each.
Answers: 3

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1

Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1

Chemistry, 23.06.2019 00:00
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3
You know the right answer?
At a certain temperature the equilibrium constant, Kc, equals 0.11 for the reaction:
2 ICl(g)...
2 ICl(g)...
Questions

Mathematics, 14.08.2020 21:01


Mathematics, 14.08.2020 21:01

History, 14.08.2020 21:01


Mathematics, 14.08.2020 21:01



Mathematics, 14.08.2020 21:01

Biology, 14.08.2020 21:01



History, 14.08.2020 21:01

Mathematics, 14.08.2020 21:01



Mathematics, 14.08.2020 21:01



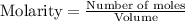
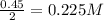
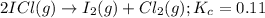
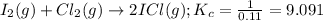
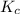 for above equation follows:
for above equation follows:![K_c=\frac{[ICl]^2}{[Cl_2][I_2]}](/tpl/images/0514/3316/221f9.png)