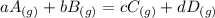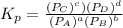Chemistry, 18.02.2020 19:34 iliketurtures
The equilibrium pressures below were observed at a certain temperature for the following reaction.
PNH₃ = 3.1 ✕ 10⁻² atm
PN₂ = 8.5 ✕ 10⁻¹ atm
PH₂ = 3.1 ✕ 10⁻³ atm
Calculate the value for the equilibrium constant at this temperature.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:10
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1
You know the right answer?
The equilibrium pressures below were observed at a certain temperature for the following reaction. <...
Questions

English, 10.02.2021 14:00

Mathematics, 10.02.2021 14:00



Mathematics, 10.02.2021 14:00


History, 10.02.2021 14:00

English, 10.02.2021 14:00

Physics, 10.02.2021 14:00

History, 10.02.2021 14:00

Mathematics, 10.02.2021 14:00

Mathematics, 10.02.2021 14:00

English, 10.02.2021 14:00

English, 10.02.2021 14:00

English, 10.02.2021 14:00




Mathematics, 10.02.2021 14:00