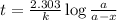Chemistry, 18.02.2020 19:16 dajmoneyp80a0o
The following reaction was monitored as a function of time: A→B+C A plot of ln[A] versus time yields a straight line with slope −4.3×10−3 /s. If the initial concentration of A is 0.260 M, what is the concentration after 225 s?

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 18:30
Two people each hold the end of a rope and create waves by moving their arms up and down. this wave is best classified as a transverse wave because a) both the rope particles and the wave are moving in the same direction. b) the wave is moving up and down as the particles of the rope move horizontally. c) the wave is moving horizontally as the particles of the rope move up and down. eliminate d) the wave is moving in a parallel direction with the motion of the person's arms.
Answers: 3
You know the right answer?
The following reaction was monitored as a function of time: A→B+C A plot of ln[A] versus time yields...
Questions



Arts, 29.04.2021 18:10

Mathematics, 29.04.2021 18:10

Mathematics, 29.04.2021 18:10



Mathematics, 29.04.2021 18:10





Mathematics, 29.04.2021 18:10


Mathematics, 29.04.2021 18:10


Mathematics, 29.04.2021 18:10



Mathematics, 29.04.2021 18:10