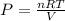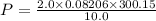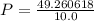Chemistry, 18.02.2020 20:57 reaganphelps3
Calculate the pressure, in atmospheres, of 2.0 mol of helium gas in a 10.0 L container at 27 degrees Celsius.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Will mark brainliest 26. which of these statements are true? (3 points) a. gases are compressible b. gases fill their containers completely c. the pressure of a gas is independent of the temperature d. gases have mass e. gases exert pressure f. the pressure of a gas is dependent on the volume g. gas pressure results from the collisions between gas particles h. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 07:00
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1
You know the right answer?
Calculate the pressure, in atmospheres, of 2.0 mol of helium gas in a 10.0 L container at 27 degrees...
Questions

Mathematics, 01.07.2019 13:30

Health, 01.07.2019 13:30



History, 01.07.2019 13:30

History, 01.07.2019 13:30


Mathematics, 01.07.2019 13:30





Mathematics, 01.07.2019 13:30

Social Studies, 01.07.2019 13:30

Mathematics, 01.07.2019 13:30

Biology, 01.07.2019 13:30

History, 01.07.2019 13:30



Mathematics, 01.07.2019 13:30