
Chemistry, 18.02.2020 21:22 leandrogarin37p2g5ds
Phosphoric acid is a triprotic acid ( K a1 = 6.9 × 10 − 3 Ka1=6.9×10−3, K a2 = 6.2 × 10 − 8 Ka2=6.2×10−8, and K a3 = 4.8 × 10 − 13 Ka3=4.8×10−13). To find the pH of a buffer composed of H 2 PO − 4 ( aq ) H2PO4−(aq) and HPO 2 − 4 ( aq ) HPO42−(aq) , which p K a Ka value should be used in the Henderson–Hasselbalch equation?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which of the following statements is true about planck’s law
Answers: 1

Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1


Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3
You know the right answer?
Phosphoric acid is a triprotic acid ( K a1 = 6.9 × 10 − 3 Ka1=6.9×10−3, K a2 = 6.2 × 10 − 8 Ka2=6.2×...
Questions

Geography, 24.08.2021 20:40





Mathematics, 24.08.2021 20:40

Mathematics, 24.08.2021 20:40


Mathematics, 24.08.2021 20:40





Computers and Technology, 24.08.2021 20:40

Mathematics, 24.08.2021 20:40

Mathematics, 24.08.2021 20:40




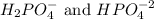 , we use the
, we use the 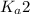
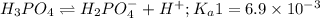
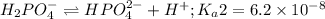
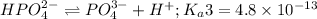
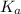 of second dissociation process
of second dissociation process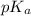 , we use the equation:
, we use the equation: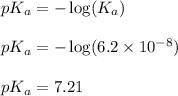
![pH=pK_a2+\log(\frac{[\text{conjugate base}]}{[\text{weak acid}]})](/tpl/images/0514/6294/e7d91.png)
![pH=pK_a2+\log(\frac{[HPO_4^{2-}]}{[H_2PO_4^-]})](/tpl/images/0514/6294/f63a6.png)
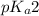 = negative logarithm of second acid dissociation constant of phosphoric acid = 7.21
= negative logarithm of second acid dissociation constant of phosphoric acid = 7.21![[HPO_4^{2-}]](/tpl/images/0514/6294/c0ca9.png) = concentration of conjugate base
= concentration of conjugate base![[H_2PO_4^{-}]](/tpl/images/0514/6294/fcc52.png) = concentration of weak acid
= concentration of weak acid

