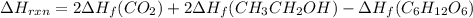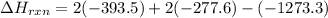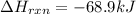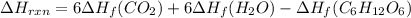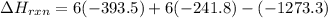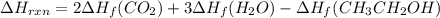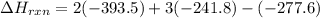Be sure to answer all parts. In winemaking, the sugars in grapes undergo fermentation by yeast to yield CH3CH2OH (ethanol) and CO2. During cellular respiration, sugar and ethanol are "burned" to water vapor and CO2.
a. Using C6H12O6 for sugar, calculate ΔH o rxn of fermentation and of respiration (combustion). Fermentation = kJ Respiration = kJ
b. Write a combustion reaction for ethanol. Include the physical states of each reactant and product. Which releases more heat from combustion per mole of C, sugar or ethanol? sugar ethanol

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3

Chemistry, 23.06.2019 03:00
Describe the properties of sodium, chlorine, and sodium chloride
Answers: 1
You know the right answer?
Be sure to answer all parts. In winemaking, the sugars in grapes undergo fermentation by yeast to yi...
Questions

History, 12.10.2019 14:50

Mathematics, 12.10.2019 14:50

Chemistry, 12.10.2019 14:50

Mathematics, 12.10.2019 14:50

Mathematics, 12.10.2019 14:50


Mathematics, 12.10.2019 14:50



Mathematics, 12.10.2019 14:50


History, 12.10.2019 14:50


Computers and Technology, 12.10.2019 14:50

English, 12.10.2019 14:50


Biology, 12.10.2019 14:50


Mathematics, 12.10.2019 14:50

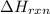 can be calculated using the standard enthalpy of formation (ΔHf) of products and reactants as follows:
can be calculated using the standard enthalpy of formation (ΔHf) of products and reactants as follows: 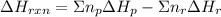 (1)
(1)