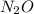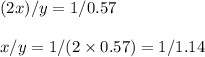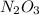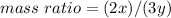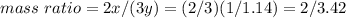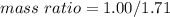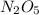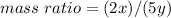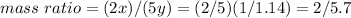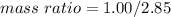Chemistry, 18.02.2020 22:23 kenndyllll
Nitrous oxide, N 2 O , has a mass ratio of nitrogen to oxygen of 1.00 : 0.57 . Determine the ratio by mass of nitrogen to oxygen in dinitrogen trioxide, N 2 O 3 , and dinitrogen pentoxide, N 2 O 5 . N 2 O 3 has a mass ratio of nitrogen to oxygen of 1.00 : N 2 O 5 has a mass ratio of nitrogen to oxygen of 1.00 :

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3
You know the right answer?
Nitrous oxide, N 2 O , has a mass ratio of nitrogen to oxygen of 1.00 : 0.57 . Determine the ratio b...
Questions


Mathematics, 27.01.2021 07:00



Chemistry, 27.01.2021 07:00

Mathematics, 27.01.2021 07:00

History, 27.01.2021 07:00



World Languages, 27.01.2021 07:00

Mathematics, 27.01.2021 07:00

Business, 27.01.2021 07:00


Mathematics, 27.01.2021 07:00

Mathematics, 27.01.2021 07:00


History, 27.01.2021 07:00