
Chemistry, 19.02.2020 00:56 rouchedavisin4
For a reaction with ΔH° = −9 kJ/mol, decide if the following statement is true. If it is false, choose the statement that makes it true. Select the single best answer. The reaction is exothermic. (Assume the reaction has a small entropy term compared to the enthalpy term) a. The statement is false. b. The reaction does not occur. c. The statement is true. d. The statement is false. e. The reaction is endergonic. f. The statement is false. g. The reaction is endothermic.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 1


Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1
You know the right answer?
For a reaction with ΔH° = −9 kJ/mol, decide if the following statement is true. If it is false, choo...
Questions

Biology, 18.11.2020 23:40



Chemistry, 18.11.2020 23:40

Social Studies, 18.11.2020 23:40

Mathematics, 18.11.2020 23:40

Mathematics, 18.11.2020 23:40

Mathematics, 18.11.2020 23:40

Mathematics, 18.11.2020 23:40

Computers and Technology, 18.11.2020 23:40

Mathematics, 18.11.2020 23:40




Mathematics, 18.11.2020 23:40

English, 18.11.2020 23:40


Chemistry, 18.11.2020 23:40

Biology, 18.11.2020 23:40

Mathematics, 18.11.2020 23:40

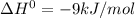 , the heat is released and is exothermic.Thus the statement that the reaction is exothermic is true.
, the heat is released and is exothermic.Thus the statement that the reaction is exothermic is true.

