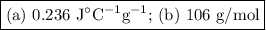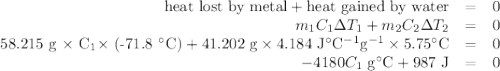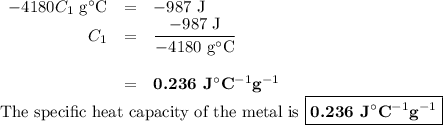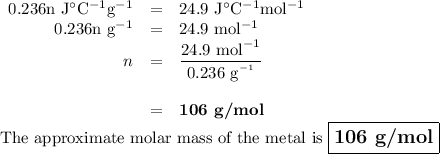Chemistry, 19.02.2020 01:04 larenhemmings
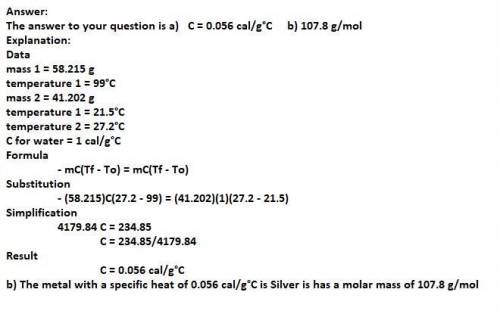
A 58.215 g sample of a pure metal is brought to 99.0c and added o 41.202 g of water at 21.5c in a calorimeter. if the metal and water arrive at a final, equal temerature of 27.2c find a) the specific heat of the metal, and b) the approximate molar mass of yhe metal

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1


Chemistry, 23.06.2019 00:20
How many lone pairs of electrons are on the central atom of no3- and what is the molecular shape? one, trigonal planar zero, trigonal pyramidal zero, trigonal planar one, tetrahedral one, trigonal pyramidal
Answers: 1

You know the right answer?
A 58.215 g sample of a pure metal is brought to 99.0c and added o 41.202 g of water at 21.5c in a ca...
Questions




English, 16.01.2020 20:31




Computers and Technology, 16.01.2020 20:31





Biology, 16.01.2020 20:31



English, 16.01.2020 20:31


Mathematics, 16.01.2020 20:31

Mathematics, 16.01.2020 20:31