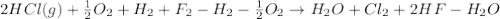Chemistry, 19.02.2020 02:23 Javanese5987
From the following enthalpies of reaction,
4 HCl (g) + O2 (g) → 2 H2O (l) + 2 Cl2 (g) ∆H = -202.4 kJ/mol 1/2 H2 (g) + ½ F2 (g) → HF (l) ∆H = -600.0 kJ/mol H2 (g) + ½ O2 (g) → H2O (l) ∆H = -285.8 kJ/mol
Calculate ∆Hrxn for 2 HCl (g) + F2 (g) → 2 HF (l) + Cl2 (g) Just input a number.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3


Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2

You know the right answer?
From the following enthalpies of reaction,
4 HCl (g) + O2 (g) → 2 H2O (l) + 2 Cl2 (g) ∆H = -2...
4 HCl (g) + O2 (g) → 2 H2O (l) + 2 Cl2 (g) ∆H = -2...
Questions

Biology, 04.06.2020 15:58

Mathematics, 04.06.2020 15:58



Mathematics, 04.06.2020 15:58


Mathematics, 04.06.2020 15:58



Spanish, 04.06.2020 15:58


Arts, 04.06.2020 15:58

Mathematics, 04.06.2020 15:58




History, 04.06.2020 15:58


Mathematics, 04.06.2020 15:58

Mathematics, 04.06.2020 15:58

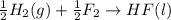 ΔH=-600.0 KJ/mol
ΔH=-600.0 KJ/mol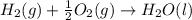 ΔH= -285.8 KJ/mol
ΔH= -285.8 KJ/mol