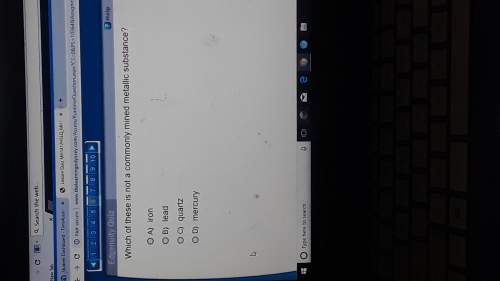
Attempt 2 A Lewis structure is a two-dimensional representation of a molecule that does not necessarily show what shape that molecule would take in three dimensions. Based on the Lewis structure and your knowledge of VSEPR theory, approximate the smallest bond angle in this molecule.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2
You know the right answer?
Attempt 2 A Lewis structure is a two-dimensional representation of a molecule that does not necessar...
Questions

Advanced Placement (AP), 12.12.2020 16:00

History, 12.12.2020 16:00


Mathematics, 12.12.2020 16:00



Mathematics, 12.12.2020 16:00



Mathematics, 12.12.2020 16:00






History, 12.12.2020 16:00

Mathematics, 12.12.2020 16:00