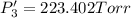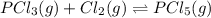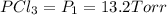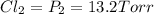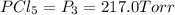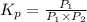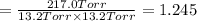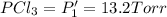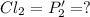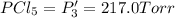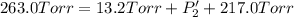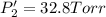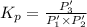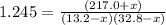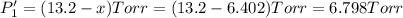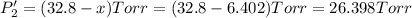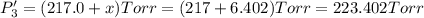Chemistry, 19.02.2020 03:25 erikabermudez55
An equilibrium mixture of PCl5(g), PCl3(g), and Cl2(g) has partial pressures of 217.0 Torr, 13.2 Torr, and 13.2 Torr, respectively. A quantity of Cl2(g) is injected into the mixture, and the total pressure jumps to 263.0 Torr (at the moment of mixing). The system then re-equilibrates. The appropriate chemical equation is:
PCl3(g) + Cl2(g) ---> PCl5(g)
Calculate the new partial pressures after equilibrium is reestablished. [in torr]
PPCl3
PPCl2
PPCl5

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

Chemistry, 23.06.2019 06:30
What type of chemical reaction occurs between silver nitrate (agno3) and copper (cu)? the equation i was given is 2agno3 + cu —> 2ag+ cu(no3)2.
Answers: 1

Chemistry, 23.06.2019 09:30
What is the force of an object when it landed(sitting in the ground)
Answers: 2

Chemistry, 23.06.2019 11:30
Distilled water is a completely neutral solution. what is its ph? a. 1 b. 7 c. 14 d. 0
Answers: 2
You know the right answer?
An equilibrium mixture of PCl5(g), PCl3(g), and Cl2(g) has partial pressures of 217.0 Torr, 13.2 Tor...
Questions


Mathematics, 23.09.2019 17:30

Health, 23.09.2019 17:30





Health, 23.09.2019 17:30

Mathematics, 23.09.2019 17:30

Social Studies, 23.09.2019 17:30

English, 23.09.2019 17:30

Health, 23.09.2019 17:30

English, 23.09.2019 17:30


Biology, 23.09.2019 17:30

Social Studies, 23.09.2019 17:30

Mathematics, 23.09.2019 17:30



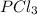 :
: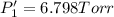
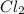 :
: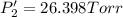
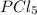 :
: