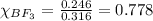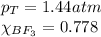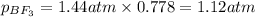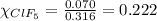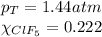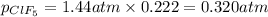Chemistry, 19.02.2020 04:02 waterborn9800
A tank at is filled with of boron trifluoride gas and of chlorine pentafluoride gas. You can assume both gases behave as ideal gases under these conditions. Calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank. Be sure your answers have the correct number of significant digits. a. boron trifluoride mole fraction:b. partial pressure:c. chlorine pentafluoride mole fraction:d. partial pressure:d. Total pressure in tank:

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3

You know the right answer?
A tank at is filled with of boron trifluoride gas and of chlorine pentafluoride gas. You can assume...
Questions

Mathematics, 21.11.2020 04:50



Mathematics, 21.11.2020 04:50


Biology, 21.11.2020 04:50



Mathematics, 21.11.2020 04:50

Mathematics, 21.11.2020 04:50

Mathematics, 21.11.2020 04:50


Engineering, 21.11.2020 04:50

History, 21.11.2020 04:50


Biology, 21.11.2020 04:50


Mathematics, 21.11.2020 04:50


Computers and Technology, 21.11.2020 04:50

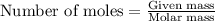 .....(1)
.....(1)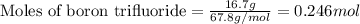
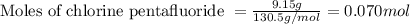
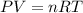
![4.19^oC=[4.19+273]K=277.19K](/tpl/images/0515/3530/8cd9a.png)
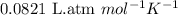
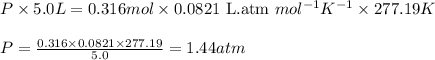
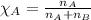 .......(2)
.......(2)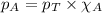 .....(3)
.....(3)