
A chemist measures the energy change during the following reaction: 1. This reaction is:. a. endothermic. b. exothermic. 2. Suppose 70.1 g of NO2 react. Will any heat be released or absorbed? A. Yes, absorbed. B. Yes, released. C. No. 3. If you said heat will be released or absorbed, calculate how much heat will be released or absorbed?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3


Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1
You know the right answer?
A chemist measures the energy change during the following reaction: 1. This reaction is:. a. endothe...
Questions

Mathematics, 26.04.2021 15:40

Computers and Technology, 26.04.2021 15:40

Social Studies, 26.04.2021 15:40

Business, 26.04.2021 15:40


Mathematics, 26.04.2021 15:40

Mathematics, 26.04.2021 15:40


Mathematics, 26.04.2021 15:40

Mathematics, 26.04.2021 15:40



Mathematics, 26.04.2021 15:40


Mathematics, 26.04.2021 15:40




History, 26.04.2021 15:40

Mathematics, 26.04.2021 15:40


 1. This reaction is:______. a. endothermic. b. exothermic.
1. This reaction is:______. a. endothermic. b. exothermic. 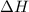 for Endothermic reaction is positive and
for Endothermic reaction is positive and 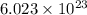 of particles.
of particles.

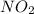 reacts, energy released = 55.3 kJ
reacts, energy released = 55.3 kJ


