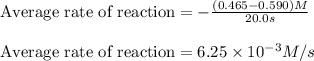Chemistry, 19.02.2020 17:28 kiaraphilman2956
In the first 22.0 s of this reaction, the concentration of HBr dropped from 0.590 M to 0.465 M . Calculate the average rate of the reaction in this time interval.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2

Chemistry, 22.06.2019 07:30
Aradio signal from a gps satellite take only about 0.067 seconds to reach a gps reciever. if the speed of light is about 300,000km/s, then approximately how far away is the reciever from from the satellite?
Answers: 1

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2

Chemistry, 22.06.2019 18:30
How many moles of lead are in 1.50 x 10^12 atoms of lead? could you explain the answer as well and not just give it to me i am refreshing for finals and i need to know how to do it
Answers: 3
You know the right answer?
In the first 22.0 s of this reaction, the concentration of HBr dropped from 0.590 M to 0.465 M . Cal...
Questions





Social Studies, 05.11.2020 19:00









Mathematics, 05.11.2020 19:00

Mathematics, 05.11.2020 19:00




Mathematics, 05.11.2020 19:00

Mathematics, 05.11.2020 19:00

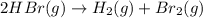
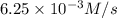
![\text{Average rate of disappearance of HBr}=-\frac{\Delta [HBr]}{\Delta t}](/tpl/images/0515/7057/16c66.png)
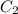 = final concentration of HBr = 0.465 M
= final concentration of HBr = 0.465 M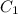 = initial concentration of HBr = 0.590 M
= initial concentration of HBr = 0.590 M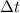 = change in time = 22.0 s
= change in time = 22.0 s