
Chemistry, 19.02.2020 18:42 rhineharttori
Given the balanced equation for the reaction of butane and oxygen: 2C4H10 + 13O2 > 8CO2 + 10H2O + energy How many moles of carbon dioxide are produced when 5.0 moles of butane react completely? * 5.0 mol 10. mol 20. mol 40. mol

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1

Chemistry, 23.06.2019 02:10
Detrimental the length of the object shown 1. 97.8mm 2. 97.80 mm 3. 97mm 4. 98mm
Answers: 2

Chemistry, 23.06.2019 02:30
Which of the four hypothetical substances you investigated would be most harmful to living organisms? 50 points!
Answers: 2

Chemistry, 23.06.2019 08:00
Problem page a jet airplane reaches 846. km/h on a certain flight. what distance does it cover in 13.0 min? set the math up. but don't do any of it. just leave your answer as a math expression. also, be sure your answer includes all the correct unit symbols.
Answers: 2
You know the right answer?
Given the balanced equation for the reaction of butane and oxygen: 2C4H10 + 13O2 > 8CO2 + 10H2O +...
Questions









Social Studies, 06.08.2019 22:10

Social Studies, 06.08.2019 22:10



Mathematics, 06.08.2019 22:10




Physics, 06.08.2019 22:10

Mathematics, 06.08.2019 22:10


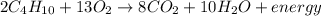
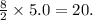 moles of carbon dioxide
moles of carbon dioxide

