

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 22.06.2019 18:30
How many moles of lead are in 1.50 x 10^12 atoms of lead? could you explain the answer as well and not just give it to me i am refreshing for finals and i need to know how to do it
Answers: 3

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1
You know the right answer?
Acetylsalicylic acid (aspirin), HC9H7O4, is the most widely used pain reliever and fever reducer. Fi...
Questions

Mathematics, 16.01.2021 01:00

English, 16.01.2021 01:00


English, 16.01.2021 01:00




Chemistry, 16.01.2021 01:00

Spanish, 16.01.2021 01:00

History, 16.01.2021 01:00

Mathematics, 16.01.2021 01:00

English, 16.01.2021 01:00



Physics, 16.01.2021 01:00

English, 16.01.2021 01:00

History, 16.01.2021 01:00

Health, 16.01.2021 01:00

Social Studies, 16.01.2021 01:00

Mathematics, 16.01.2021 01:00


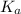 for above equation follows:
for above equation follows:![K_a=\frac{[C_9H_7O_4^-][H^+]}{[HC_9H_7O_4]}](/tpl/images/0515/8851/cb0ef.png)

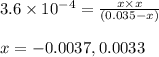
 = x = 0.0033 M
= x = 0.0033 M![pH=-\log[H^+]](/tpl/images/0515/8851/cf945.png)



