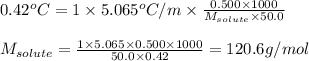The molecular weight of an organic compound was determined by measuring the freezing point depression of a benzene solution. A 0.500 g sample was dissolved in 50.0 g of benzene, and the resulting depression was 0.42°C (kf (benzene) = 5.065°C/m). What is the approximate molecular weight of the compound?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3

Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 7.3 × 106 m/s. show your work. note: h = plank's constant (6.62607 x 10-34 j s)
Answers: 1

Chemistry, 23.06.2019 05:30
For the reaction i2(g)+br2(g)←−→2ibr(g), kc=280 at 150 ∘c. suppose that 0.450 mol ibr in a 2.00-l flask is allowed to reach equilibrium at 150 ∘c. what is the equilibrium concentration of 2ibr, i2, br2
Answers: 1
You know the right answer?
The molecular weight of an organic compound was determined by measuring the freezing point depressio...
Questions


Biology, 23.07.2019 09:10





English, 23.07.2019 09:10

Chemistry, 23.07.2019 09:10




Chemistry, 23.07.2019 09:10





Physics, 23.07.2019 09:10



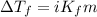
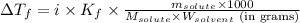
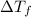 = depression in freezing point = 0.42°C
= depression in freezing point = 0.42°C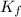 = molal freezing point elevation constant = 5.065°C/m
= molal freezing point elevation constant = 5.065°C/m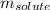 = Given mass of solute (sample) = 0.500 g
= Given mass of solute (sample) = 0.500 g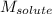 = Molar mass of solute (sample) = ? g/mol
= Molar mass of solute (sample) = ? g/mol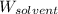 = Mass of solvent (benzene) = 50.0 g
= Mass of solvent (benzene) = 50.0 g