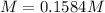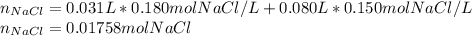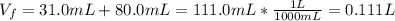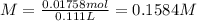Chemistry, 19.02.2020 20:04 taridunkley724
Calculate the molarity of the solution produced by mixing 31.0 mL of 0.180 M NaCl and 80.0 mL of 0.150 M NaCl. (Assume that the volumes are additive.)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1

Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2
You know the right answer?
Calculate the molarity of the solution produced by mixing 31.0 mL of 0.180 M NaCl and 80.0 mL of 0.1...
Questions



Mathematics, 30.03.2021 01:30


Mathematics, 30.03.2021 01:30

Biology, 30.03.2021 01:30

Chemistry, 30.03.2021 01:30

Mathematics, 30.03.2021 01:30

Mathematics, 30.03.2021 01:30


Spanish, 30.03.2021 01:30

Business, 30.03.2021 01:30


History, 30.03.2021 01:30


Mathematics, 30.03.2021 01:30


Chemistry, 30.03.2021 01:30


Mathematics, 30.03.2021 01:30