

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3


Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2
You know the right answer?
The bond enthalpy of the oxygen-oxygen bond in O2 is 498 kJ/mol. Based on the enthalpy of the reacti...
Questions


Mathematics, 12.02.2020 21:21


Computers and Technology, 12.02.2020 21:22


Mathematics, 12.02.2020 21:22














History, 12.02.2020 21:23

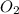 is 498 kJ / mol. What is the average O=O bond energy of the bent ozone molecule O=O=O?
is 498 kJ / mol. What is the average O=O bond energy of the bent ozone molecule O=O=O?
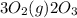
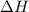 for 2 moles of ozone is
for 2 moles of ozone is 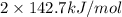 = 285.4 kJ/mol.
= 285.4 kJ/mol.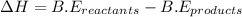
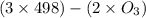
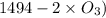
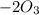
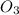 = 604.3 kJ/mol
= 604.3 kJ/mol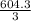 kJ/mol
kJ/mol

