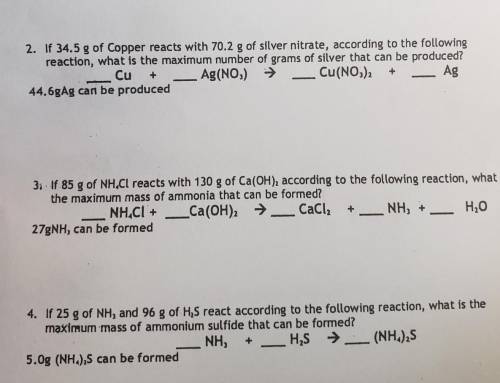
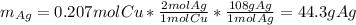If 34.5 g of Copper reacts with 70.2 g of silver nitrate, according to the following
reaction,...

Chemistry, 19.02.2020 22:04 ammyraammirez24
If 34.5 g of Copper reacts with 70.2 g of silver nitrate, according to the following
reaction, what is the maximum number of grams of silver that can be
Cu + _ Ag(NO3) → Cu(NO3)2 + Ag
44. 6gAg can be produced

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:40
What type of solution is formed if 10 g of kclo3 are dissolved in 100g of water at 30
Answers: 2

Chemistry, 21.06.2019 22:00
The graph above shows how the price of cell phones varies with the demand quantity. the equilibrium price for cell phones is where both supply and demand quantities equal $100, 5,000 5,000, $100
Answers: 2

Chemistry, 22.06.2019 00:00
The p sub shell can hold up to 8 electrons in an atom. true or false?
Answers: 1

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1
You know the right answer?
Questions













Physics, 05.12.2019 02:31





History, 05.12.2019 02:31

Computers and Technology, 05.12.2019 02:31

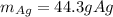
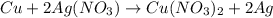
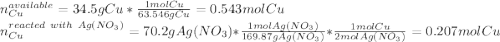 In such a way, since there are more available moles of copper, it is in excess, so the silver nitrate is the limiting reactant, this, the maximum grams of silver result:
In such a way, since there are more available moles of copper, it is in excess, so the silver nitrate is the limiting reactant, this, the maximum grams of silver result: